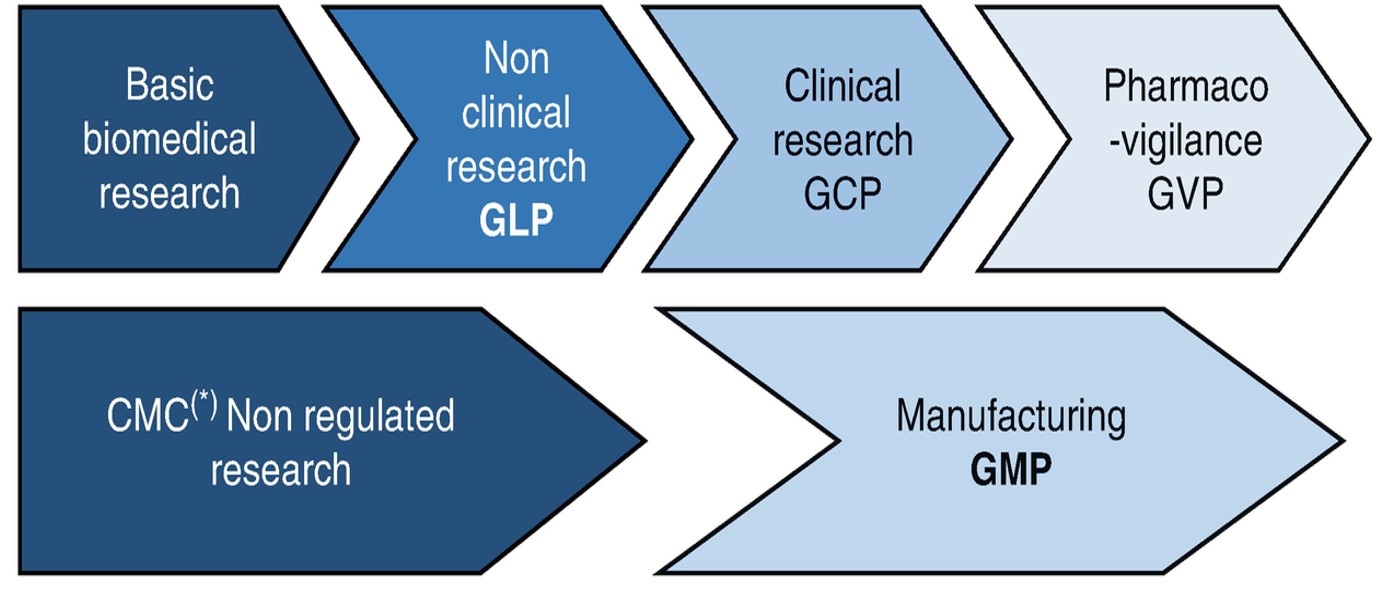
Drug Regulatory Affairs gives awareness in the aspects of Regulatory and Quality Compliance in the pharmaceutical industry, National and International Drug Approvals & Bio-ethics, Research Methodology & Pharmacological Screening, Modern Analytical Techniques, Clinical Trials & Healthcare Policies, National Regulatory Affairs, International Regulatory Systems, Emerging Concept in Regulatory Affairs, GLP, GMP & Validation, Intellectual Property Rights & Bioethics. In an overall the basics principles of Regulatory Affairs, GLP, IPR and Bioethics in Clinical Research are covered.